Epigenetics-Based Therapeutics: A New Era in Precision Medicine
Introduction
In recent years, the field of precision medicine has witnessed remarkable advancements, driven by cutting-edge technologies and a deeper understanding of human biology. Among the most exciting frontiers is epigenetics-based therapeutics, an innovative approach that promises to revolutionize how we diagnose, treat, and prevent diseases. This article explores the fundamental concepts of epigenetics, how epigenetic modifications influence health and disease, and the emerging therapeutic strategies that harness this knowledge to usher in a new era of personalized medicine.
Definition
Epigenetics-based therapeutics are treatments that target the epigenetic mechanisms – such as DNA methylation, histone modification, and non-coding RNA regulation – that control gene expression without altering the underlying DNA sequence. By reversing abnormal epigenetic changes associated with diseases like cancer, neurological disorders, and autoimmune conditions, these therapies aim to restore normal gene function and improve health outcomes.
Understanding Epigenetics: Beyond DNA Sequence
Traditionally, genetics has focused on the DNA sequence – the “blueprint” of life. However, epigenetics refers to heritable changes in gene expression that do not involve alterations to the underlying DNA sequence. These changes are mediated by chemical modifications that regulate how genes are turned on or off. Key epigenetic mechanisms include:
- DNA Methylation: Addition of methyl groups to DNA molecules, usually suppressing gene expression.
- Histone Modifications: Chemical changes to histone proteins around which DNA is wrapped, influencing chromatin structure and gene accessibility.
- Non-coding RNAs: RNA molecules that can regulate gene expression at various levels.
These epigenetic marks are dynamic and responsive to environmental factors such as diet, stress, toxins, and lifestyle, making them a critical interface between genes and environment.
The Role of Epigenetics in Health and Disease
Epigenetic regulation is essential for normal development, cellular differentiation, and maintaining homeostasis. However, aberrant epigenetic changes can lead to the malfunctioning of genes and contribute to the onset and progression of various diseases, including cancer, neurological disorders, autoimmune diseases, and cardiovascular conditions.
For example:
- In cancer, abnormal DNA methylation can silence tumor suppressor genes, allowing uncontrolled cell growth.
- In neurodegenerative diseases like Alzheimer’s, altered histone acetylation patterns impact neuron function and survival.
- Autoimmune disorders may involve epigenetic dysregulation that affects immune cell behavior.
Recognizing these epigenetic alterations as disease drivers opens the door for therapeutic interventions aimed at reversing or modifying these changes.
Epigenetics-Based Therapeutics: Precision Medicine in Action
Epigenetics-based therapeutics involve drugs or treatment strategies designed to target and modulate epigenetic marks, thereby restoring normal gene function or altering disease pathways. This approach aligns perfectly with the goals of precision medicine, which seeks to tailor treatments based on individual genetic and molecular profiles.
Types of Epigenetic Drugs
Several classes of epigenetic drugs have emerged, with many already approved or in clinical trials:
- DNA Methyltransferase Inhibitors (DNMTi):
These agents block enzymes that add methyl groups to DNA, leading to reactivation of silenced genes. Examples include azacitidine and decitabine, used primarily in hematologic cancers like myelodysplastic syndrome. - Histone Deacetylase Inhibitors (HDACi):
By inhibiting enzymes that remove acetyl groups from histones, HDAC inhibitors promote a more open chromatin structure and increased gene expression. Drugs like vorinostat and romidepsin have been approved for treating certain lymphomas. - Histone Methyltransferase and Demethylase Inhibitors:
Targeting enzymes that add or remove methyl groups from histones offers another level of control, with ongoing research identifying promising compounds for cancer and other diseases. - Non-coding RNA Therapeutics:
Emerging strategies use synthetic RNAs or RNA inhibitors to modulate gene expression, offering potential treatments for diseases with epigenetic underpinnings.
Advantages of Epigenetics-Based Therapeutics
- Reversibility: Unlike genetic mutations, epigenetic modifications are reversible, allowing dynamic therapeutic intervention.
- Target Specificity: Drugs can be designed to target specific epigenetic enzymes or pathways implicated in particular diseases, reducing off-target effects.
- Combination Therapy Potential: Epigenetic drugs can be combined with conventional therapies (chemotherapy, immunotherapy) to enhance efficacy and overcome resistance.
- Broad Application: Epigenetic mechanisms are involved in diverse diseases, expanding the therapeutic possibilities beyond cancer to neurological and metabolic disorders.
Current Clinical Applications and Success Stories
Epigenetics-based therapeutics have already made a significant impact in oncology, a field where abnormal epigenetic landscapes are well documented.
- Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML): Azacitidine and decitabine have improved survival rates by reactivating silenced genes that control cell growth and apoptosis.
- Cutaneous T-Cell Lymphoma: Vorinostat and romidepsin are effective in patients resistant to conventional treatments.
- Emerging Trials: Epigenetic drugs are being tested in solid tumors such as lung, breast, and prostate cancer, as well as in non-oncological diseases like Fragile X syndrome and certain types of epilepsy.
Challenges and Future Directions
Despite their promise, epigenetics-based therapeutics face several challenges:
- Complexity of Epigenetic Regulation: The epigenome is highly dynamic and context-dependent, making it difficult to predict responses and design universal treatments.
- Biomarker Development: It’s crucial to find trustworthy epigenetic biomarkers for patient selection and therapy response tracking.
- Off-Target Effects: Epigenetic drugs can affect multiple genes and pathways, sometimes leading to unintended consequences.
- Drug Resistance: Like other therapies, resistance to epigenetic drugs can develop, necessitating new strategies.
Future research is focused on:
- Developing more selective and potent epigenetic modulators.
- Combining epigenetic therapy with immunotherapy to enhance immune response against tumors.
- Utilizing CRISPR-based epigenome editing for precise and locus-specific epigenetic modifications.
- Expanding understanding of epigenetics in aging and chronic diseases to develop novel interventions.
The Promise of Personalized Epigenetic Medicine
The ultimate vision of epigenetics-based therapeutics is to integrate multi-omics data – including genomics, epigenomics, transcriptomics, and proteomics – to create comprehensive molecular profiles for patients. This approach allows clinicians to:
- Predict disease risk based on epigenetic patterns.
- Customize treatment regimens with targeted epigenetic drugs.
- Monitor therapy effectiveness through epigenetic biomarkers.
- Adjust interventions dynamically as the epigenome changes over time.
Such personalized epigenetic medicine could transform healthcare by improving outcomes, minimizing side effects, and reducing healthcare costs.
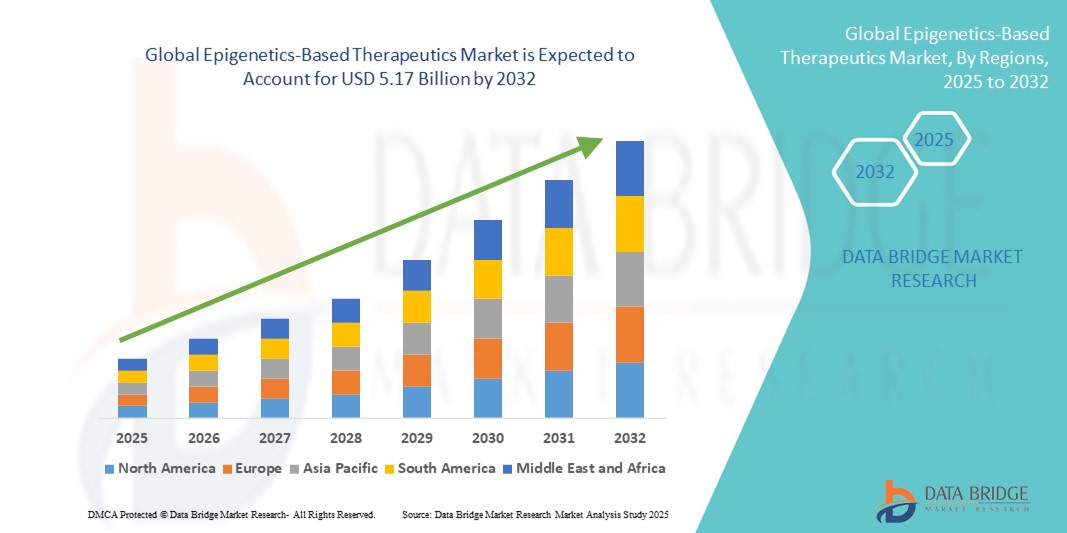
Growth Rate of Epigenetics-Based Therapeutics Market
According to Data Bridge Market Research, at a compound annual growth rate (CAGR) of 14.40 percent, the global epigenetics-based therapeutics market is projected to grow from its 2024 valuation of USD 1.76 billion to USD 5.17 billion by 2032.
Learn More: https://www.databridgemarketresearch.com/reports/global-epigenetics-based-therapeutics-market
Conclusion
Epigenetics-based therapeutics represent a groundbreaking advance in precision medicine, offering new hope for treating complex diseases by targeting the regulatory mechanisms that control gene expression. With ongoing research and technological innovation, this field is rapidly evolving from bench to bedside. As we unlock the secrets of the epigenome, we move closer to a future where therapies are tailored to each individual’s unique molecular landscape – truly realizing the promise of personalized medicine.




